Find a Study
The FTD Disorders Registry helps to support clinical trials in all forms of frontotemporal degeneration, including:
- Behavioral variant frontotemporal degeneration (dementia) (bvFTD)
- Semantic variant primary progressive aphasia (svPPA)
- Nonfluent/agrammatic variant PPA (naPPA)
- Logopenic variant PPA (lvPPA)
- Progressive supranuclear palsy (PSP)
- Corticobasal degeneration (CBD) / corticobasal syndrome (CBS)
- Frontotemporal degeneration with amyotrophic lateral sclerosis (FTD-ALS)
Terms used on this page:
-
Observational Study: A study that collects information about participants’ health or behavior without testing a specific treatment or intervention.
-
Treatment Study: A study that tests how well a drug, therapy, or other intervention works for people with a certain condition.
-
In-Person: Participation requires visits to a clinic, hospital, or research site.
-
Remote: Participation can be done from home, usually via a survey, online meeting or phone call.
Current Studies
Observational Studies (Natural History or Longitudinal)
The ASSESS ALL ALS Study is recruiting individuals both with and without ALS to participate in a long-term study tracking the progression of ALS and collecting biological samples such as blood and cerebrospinal fluid. ASSESS enables individuals living with ALS to participate remotely or at one of our active sites, minimizing the need to travel to a clinical location. This study will set a new standard for how we understand ALS by gathering data from a large group of participants that can then be accessed by researchers nationwide.
Any individual living with ALS and who meets study inclusion/exclusion criteria may participate either onsite at one of our activated study sites or remotely. Symptomatic participants who choose to participate onsite at a clinic will be seen approximately every 4 months. Symptomatic participants who choose to participate remotely will complete remote visits every 4 months from their home using a personal device to complete questionnaires and will have blood drawn by an in-home phlebotomist. Control participants will be seen approximately every 12 months onsite.
During these visits participants will undergo clinical assessments including:
- the ALS Functional Rating Scale-Revised
- Handheld Dynamometry
- Vital Capacity
Participants will also complete cognitive testing and biofluid samples, including blood and cerebrospinal fluid, will be collected. Participants will also be expected to complete monthly digital speech assessments and electronic questionnaires. A stipend will be given to participants after the completion of each study visit.
More information about ALL ALS can be found on the ALL ALS website or by emailing the Patient Navigation Team at info@all-als.org.
The Frontotemporal Degeneration Centerand the Linguistic Data Consortium at the University of Pennsylvania are working to develop simple, easy, and effective ways to track neurocognitive health through short interactions with a web app. You can help us create a large open dataset, which researchers all over the world will use to create and test automated methods to track neurocognitive health.
Today, diagnosing neurodegenerative disorders like Alzheimer’s Disease or Frontotemporal Degeneration depends on someone scoring below a low threshold on clinically administered tests, which often means that they’ve been suffering from the disease for some time. Often the time to diagnosis can be a decade or more if you live remotely or the condition has an uncommon clinical presentation. Easy and reliable tracking over time, including remote monitoring, will let us identify early changes and allow more timely diagnosis, and to see how neurocognitive health changes over time. The same methods will support large-scale evaluation of interventions, whether those are medications or lifestyle changes.
This project is not just aimed at participants with cognitive impairment, or even at elderly people — we need all ages and backgrounds and conditions. The tasks involve oral descriptions of two pictured scenes, and naming as many words as you can in a minute that are from a target category. We will publish an anonymized version of the data for use by research groups worldwide.
To participate in the first step of this project, which should take less than five minutes of your time, click here.
Have you recently had difficulties with planning, organizing, or solving problems? Changes in personality or mood that affect your daily life? Problems managing or completing task you’ve done before? If you are between the ages of 40 and 64, and are concerned about your thinking or memory ability, the BEYONDD study might be right for you. Volunteers from diverse populations (Latinx/Hispanic, Black/African American, Asian American, Pacific Islander, and American Indian/Alaska Native) are needed to help researchers improve brain health in these groups.
BEYONDD is a diverse team of doctors, scientists, and other researchers, working together to bring diversity and inclusion to early onset dementia (EOD) research by reaching out to adults from diverse populations. EOD impacts people at the peak of life. It changes the way people think, act, talk, and behave. Little is known about EOD in diverse populations.
Join the study looking to lower the health disparities in the U.S. and make sure that people of all races are included in finding new ways to treat EOD. Study volunteers get some amazing perks. You’ll get to work with world-renowned experts and learn more about your own health. You’ll have access to digital tests of thinking and memory, clinical laboratory tests, and feedback from the clinical team – all from the comfort of your own home. If you are able and willing to come into one of BEYONDD’s expert centers after completing the online study, you can access a one-on-one session with a BEYONDD doctor and brain scans. Even better, it’s all at no cost to you.
For more information, visit www.beyonddproject.org or call us at 1 (866)-7MYMIND.
This study is enrolling children and young adults from families with frontotemporal dementia (FTD) and Alzheimer’s disease (AD) caused by genetic variants. For FTD, these include MAPT, GRN, and C9orf72.
The purpose of this study is to better understand brain development in families with dementia. Brain development in persons from genetic FTD and AD families will also be compared to persons from neurodiverse populations.
Participation involves a 2- to 3-day visit to the University of California, San Francisco. This visit includes clinical and cognitive evaluation, MRI brain scan, emotions testing, questionnaires, and a saliva sample for genetic testing. Participants and their family members will not learn their mutation status.
Participants must be between 7 and 17 years old (young adults up to 25 may be eligible), have a biological family member with genetic FTD or AD, and have a study partner who can accompany them.
Travel, lodging, and meals will be reimbursed; and monetary compensation for the study will be provided.
Target enrollment is 190 participants.
This online study is being conducted by the University of California San Francisco Memory and Aging Center to learn more about changes in decision-making in family members of patients with genetic forms of FTD.
It compares decision-making in family members who have these genes to family members who don’t have these genes.
You are eligible to take part in this study if you are a family member of a patient with a genetic form of FTD that is enrolled in the ARTFL-LEFFTDS Longitudinal Frontotemporal Lobar Degeneration (ALLFTD) Study, and you yourself do not have a diagnosis of dementia.
The Alzheimer Disease Related Dementia studies at the University of Miami are looking for participants to better understand the genetic factors contributing to frontotemporal dementia (FTD), especially in Hispanic, African American, and Native American populations.
Genetics helps us improve the knowledge of biology, diagnosis, and future therapies for these diseases.
Participants must be 18 years or older; have a diagnosis of FTD, PPA, or semantic dementia; and identify as Hispanic, African American, or Native American.
Participation includes interviews for family and medical history and clinical assessments, which can be done remotely in one or two visits (total about 3 hours), as well as a one-time blood draw, which can be completed at the participant's home.
Target enrollment is 100 participants.
For more information, email Anisley Martinez at FTD-HIHG@miami.edu or visit the University of Miami Frontotemporal Dementia Research webpage.
The Genetic Frontotemporal dementia Initiative (GENFI) is a group of research centres across Europe and Canada with expertise in familial frontotemporal dementia (FTD), and is co-ordinated by Professor Jonathan Rohrer at University College London.
The aim of the study is to understand more about genetic FTD, particularly in individuals who have mutations in the progranulin (GRN), microtubule-associated protein tau (MAPT) and chromosome 9 open reading frame 72 (C9orf72) genes.
GENFI investigates both people who have developed symptoms and also people who have a risk of developing symptoms in the future because they carry an abnormal genetic mutation. By studying individuals who are genetically predisposed to develop the disease later in life, we gain insights into the earliest changes in the development of the disease. The key objectives of GENFI are therefore to develop markers which help identify the disease at its earliest stage, as well as markers that allow the progression of the disease to be tracked.
The GENFI consortium currently consists of sites across the UK, Netherlands, Belgium, France, Spain, Portugal, Italy, Germany, Switzerland, Sweden, Denmark, Finland, Croatia, Serbia, Turkey and Canada. See the full list of sites here.
Researchers, led by Dr. Elizabeth Finger, and funded by the Canadian Institutes for Health Research, are studying brain development in children from families with frontotemporal degeneration (FTD) caused by an FTD-associated genetic variants, such as microtubule associated protein tau (MAPT), progranulin (known as granulin or GRN), or chromosome 9 open reading frame 72 (C9ORF72).
Contact Information: Please contact the study coordinator at Kristy Coleman, at cognitiveneurology@sjhc.london.on.ca or 519-646-6100 if you would like to participate in this study or have further questions.
What is the GENFI-NeuroDev study and its purpose?
The GENFI-NeuroDev study is an extension of the Genetic FTD Initiative (GENFI). It aims to understand brain development in youth aged 9 to 17 who have a family history of hereditary (genetic) frontotemporal degeneration (FTD) in a first or second degree relative.
Why are they doing this study?
FTD has always been considered an adult-onset neurodegenerative disease. The hereditary form of FTD is linked to different genes, with the three most common being C9orf72, GRN, and MAPT. These genes have critical roles during key periods of brain development, which happens until around 30 years old. This raises the question of whether brain development is affected by FTD-causing genes. Learning about how brain development in youth who do not have FTD can help identify new potential targets for FTD treatment.
Who can participate?
- Youths ages of 9 to 16 years at time of enrollment who have a biological parent or grandparent who has tested positive for an FTD causing gene.
- Parents or guardians of potential GENFI NeuroDev participants are all aware of the autosomal dominant genetic nature of FTD in their family prior to being approached for potential participation in this study. However, parents or grandparents do not need to know their own genetic status, and children will not undergo clinical genetic testing.
What would participating entail?
Timeline: Participants will have an initial first visit, and then if interested, a follow-up visit 2 years later.
Testing involved: A neurological exam, cognitive and behavioural assessments, MRI scan, and collection of blood (optional) or saliva for genetic testing.
Time needed per visit: 3-4 hours
Preparation before visit: None
Costs: None
Compensation: Participants will be reimbursed for travel-related expenses, including compensation for time.
Where is this study located?
- Western University, London, Canada
- McGill University, Montreal, Canada
- Laval University, Quebec City, Canada
- Sunnybrook Hospital, Toronto, Canada
- Karolinska Institute, Stockholm, Sweden
- University College London, London, United Kingdom
- University of Halle, Halle, Germany
- University of Tubingen, Tubingen, Germany
- Erasmus University Rotterdam, Rotterdam, Netherlands
I don’t live in one of the cities that conducts this study, but I’m interested. Who do I contact?
Please contact the study coordinator, Kristy Coleman, at
cognitiveneurology@sjhc.london.on.ca or 519-646-6100, who will be able to provide more information and direct you to a nearest participating site for more options.
The observational primary progressive aphasia (PPA) research program at Northwestern University seeks to study individuals living with PPA over time using neuropsychological testing and advanced imaging techniques.
Participants are asked to come to Chicago in order to help:
- understand progression in PPA and its link to brain changes,
- increase awareness of PPA and better educate patients, families, and clinicians,
- identify biomarkers that will lead to earlier diagnosis and earlier intervention.
Participants are compensated for participation and travel expenses, and meals are covered for those not local to Chicago.
For more information, please see the clinical trials.gov listing or join our registry.
Researchers at the Mayo Clinic are currently enrolling patients to participate in an observational study aimed at learning more about Primary Progressive Apraxia of Speech, Primary Progressive Aphasia and other related disorders. The study involves traveling to Mayo Clinic in Rochester, MN on a yearly basis to complete tests designed to help researchers learn more about these diseases and how they affect the brain over time. Participants will see a Neurologist, Speech Language Pathologist and have imaging scans like MRI, FDG PET, Amyloid PET, Tau PET and/or Neuroinflammation PET. All of the tests and doctor visits are covered by the study.
If you’re interested in learning more, please contact Sarah Boland, CCRP at boland.sarah@mayo.edu.
Have you or a loved one been diagnosed with an FTD disorder, dementia or Parkinson’s?
Nutritional intake can be a challenge. Help researchers better understand your needs! Individuals and their care partners are invited to participate in an in-home research study investigating the eating patterns of people with dementia. You may qualify if you currently live at home in Oregon. Volunteers will be compensated and offered free nutritional and in-home swallowing assessments
For more information, contact: (541) 346-7494 or eatinglab@uoregon.edu
.
Researchers from Emory University are seeking participants for a 15-minute survey.
To qualify, you must:
- Have a family history of ALS, FTD, Ataxia or Huntington’s disease
- Currently have no symptoms of ALS, FTD, Ataxia or Huntington’s disease
You must also have either:
- A family member with a known mutation for any of the conditions described above
AND/OR
- A known mutation yourself for any of the conditions described above
To access the survey, please click here.
Yale PET Center is studying behavioral variant frontotemporal dementia (bvFTD) with the new synaptic density positron emission tomography (PET) tracer 18F-SynVesT-1. The new tracer binds to a protein in synapses and provides synaptic measurements in living people. By comparing this to a known PET tracer, 18F-FDG, this study seeks to provide early detection and progression of bvFTD. Participation involves one phone screening and at least three in-person visits to sites in New Haven, Connecticut. In-person visits include a screening appointment, a magnetic resonance imaging (MRI) scan, and up to two PET scanning sessions. The study will recruit 15 persons between the ages of 40 and 80 who have been diagnosed with bvFTD. For more information, contact Ebrahimian Sadabad, Faranak MD by email
faranak.ebrahimiansadabad@yale.edu or by phone at 203-785-5054
The goal of this study is to identify the most reliable methods of analysis for tracking CBD, PSP and o/vPSP over time. The results from this study may be used in the future to calculate statistical power for clinical drug trials. The study will also provide information about the relative value of novel imaging techniques for diagnosis, as well as the value of imaging techniques versus testing of blood, urine and cerebrospinal fluid (CSF) ‟biomarkers.”
Study Details
Inclusion Criteria: Participants must be between the ages of 40 and 80. They must also have no known history of neurological disease, or meet criteria for one of the following: corticobasal syndrome or degeneration (CBS or CBD); progressive supranuclear palsy (PSP); or oligo- or variant-progressive supranuclear palsy (o/vPSP). Participants will need a reliable study partner who has frequent contact with the participant, who is available to provide information about the participant, and who can accompany the participant to research visits as needed. All participants must be willing and able to undergo testing procedures, which include longitudinal follow-up visits. Participants must be able to walk five steps with minimal assistance.
Exclusion Criteria: Other than those listed above, any significant neurological disease, including Parkinson’s disease, multi-infarct dementia, Huntington’s disease, normal-pressure hydrocephalus, brain tumor, seizure disorder, subdural hematoma and multiple sclerosis. Also exclusionary is a history of significant head trauma followed by persistent neurological deficits or known structural abnormalities. Any significant systemic illness or unstable/uncontrolled medical condition is exclusionary. The presence of pacemakers, aneurysm clips, artificial heart valves, ear implants, or metal fragments or metal objects in the eyes, skin, or body is exclusionary. No longstanding history (longer than 10 years) of alcohol or substance abuse with continuous abuse up to and including the time that the symptoms leading to clinical presentation developed. No longstanding history (longer than 10 years) of major depression, bipolar disorder or schizophrenia that has had continuous or intermittent symptoms similar to the clinical presentation and that required medication control up until the time of evaluation. No clinically significant abnormalities in B12, RPR or TFTs that may interfere with the study are exclusionary. A participant is also ineligible if the study director deems that the participant will be unable to complete sufficient key study procedures; exceptions to these guidelines may be considered on a case-by-case basis by the study director.
What to Expect
Testing: Participants may be asked to undergo the following testing procedures, though not all participants will be asked to undergo every procedure listed: neurological and physical examinations; interviews with the study partner; MRI scans of the brain; PET scans of the brain; eye movement testing; retinal imaging; memory & thinking testing, daily-function assessments; computer-based tests of decision-making and executive abilities; collection of a detailed family history; blood and urine collection for analysis and storage/banking; behavioral testing; speech and language evaluations; mood questionnaires; and optional lumbar punctures (LPs), either conventional and CT-guided, to collect cerebrospinal fluid (CSF) for analysis and storage.
The Frequency of Visits: All participants will be asked for one visit every six months for 12 months (i.e., a total of three visits over one year). o/vPSP and healthy volunteer participants will be asked for an additional visit approximately 12 months after the third visit (i.e., three visits over the first year and then one additional visit two years after the start of the study, for a total of four visits over two years). MRI brain scans will occur at the UCSF Neuroscience Imaging Center. PET brain scans will occur at Lawrence Berkeley National Laboratory. The remainder of the study procedures will occur at the UCSF Memory and Aging Center or the UCSF Neurosciences Clinical Research Unit.
Materials Needed Before Evaluation: Medical records and MRI scans (if available).
Costs: No costs will be charged for any of the study procedures. In return for time, effort and travel expenses, participants will receive $40 for each visit completed, $50 for each MRI brain scan, $50 for the PiB PET scan, $100 for each AV1451 PET scan and $100 for every lumbar puncture.
Contact Information
If you are interested in participating in this study or have any questions, please contact the study coordinator, Rebecca Snell, at Rebecca.Snell@ucsf.edu.
Researchers at the University of California, San Francisco Memory and Aging Center are conducting a study to better understand and diagnose Primary Progressive Aphasia (PPA) in Chinese-speaking individuals. This study will develop and validate the first comprehensive Chinese Language Assessment Battery for Primary Progressive Aphasia (CLAP).
The study involves comprehensive language assessments, brain imaging (MRI), cognitive testing, and blood sample collection. Some participants will be asked to return for follow-up assessments to track changes over time. This research will help improve diagnosis and care for Chinese-speaking individuals with PPA, a group that has been historically underrepresented in medical research.
Who may be eligible to participate?
· Must be a native Chinese speaker
· Must be diagnosed with PPA/FTD OR be a healthy volunteer
Participants will undergo:
· Specialized Chinese language assessments (available in both Mandarin and Cantonese)
· Brain imaging (MRI)
· Cognitive testing
· Blood sample collection
· Optional follow-up visits over the five-year study period
Travel and accommodation costs will be covered for both the participant and one companion.
Note: Assessments are also available in English, Spanish, and Tagalog for multilingual participants.
For more information, please contact: (415) 514-6543 | kassey.chang@ucsf.edu
The purpose of this research program is to develop a better understanding of the causes and natural course of FTD and related neurodegenerative diseases by creating a large collection of different types of data. These diseases cause damage to brain cells (neurons), and they slowly worsen over time. They may cause problems with thinking, memory, movement, language, and behavior. The data and samples collected in this study are analyzed for several ongoing NIH funded studies and will be stored securely for future use.
Participation in research has an enormous impact on advancing the clinical care for FTD and related disorders, The data and biological samples collected allow us to study the biology of FTD and related disorders over time and contributes to improving diagnosis, developing new markers of prognosis, and discovering therapeutic targets that can enhance and further the development of treatment trials aimed at treating the underlying biology of FTD and related disorders. We collaborate on these projects with other neurodegenerative disease centers within Penn, nationally, and internationally, and the knowledge we gain helps collaborators worldwide move the scientific understanding of disease forward to improve diagnosis and treatment for all impacted by FTD.
What am I expected to do in this study?
- Annual study visits for 3 years (if possible)
- Study activities can vary and may include:
-Lumbar puncture
-MRI (3T)
-Cognitive testing
-Neurological exam
-Speech tasks
-Blood draw
-Family history collection
-Medical history collection
-Questionnaires
For more information, contact: Emily Xie at Emily.xie@pennmedicine.upenn.edu or visit ClinicalTrials.gov.
The Laboratory of Cognition and Neural Stimulation (LCNS) at the University of Pennsylvania is recruiting both individuals with Primary Progressive Aphasia and healthy individuals to participate in a research study examining the connection between language and hand gestures in Primary Progressive Aphasia.
This study will examine brain activity using a technique called electroencephalography (EEG). Participants will wear a fabric cap on the head which holds small metal discs called electrodes. Electrodes will also be attached to face with stickers. EEG has been used for many years and is considered a safe procedure; it does not cause any pain.
Note: This is not a treatment study, and you should not expect your condition to change by joining the study.
Who is eligible?
Individuals must:
- Be between ages 45 - 80 years old
- Be native or near-native proficiency in speaking, understand, and reading English
- Have normal vision or corrected to normal vision (e.g., glasses or contact lenses)
- Have normal hearing or corrected to normal hearing (e.g., hearing aid)
- Be able to travel to our facility located near the Hospital of the University of Pennsylvania in Philadelphia
You must not:
- Have a history of neurological disorders or brain injury (other than Primary Progressive Aphasia)
- Have a history of severe psychiatric disorders
- Have a history of alcohol or drug abuse
Visit Breakdown
Visit 1: EEG Recording Session (2 hr)
Visit 2: EEG Recording Session (2 hr)
Visit 3: Behavioral Testing Session (2 hr)
Compensation
- $25.00 per hour for each session
- Reimbursement for transportation costs (up to $50.00 per session)
More Information
If you are interested in participating, please contact: Dr. Amy Lebkuecher via email at Amy.Lebkuecher@pennmedicine.upenn.edu or by phone at 215-703-7052
Treatment Studies
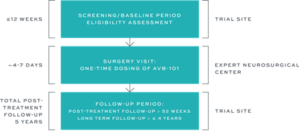
ASPIRE-FTD is a Phase 1/2 open-label, multi-center study designed to evaluate the safety and preliminary efficacy of AVB-101 in patients with FTD-GRN. AVB-101 is an investigational one-time therapy designed to deliver a functional copy of the progranulin (GRN) gene directly to the brain, thereby potentially restoring progranulin levels and stopping disease progression in patients with FTD-GRN. ASPIRE-FTD is the first study of AVB-101 in humans. It aims to evaluate the safety of AVB-101 and measure its effects on progranulin levels and the symptoms of FTD.
WHO CAN PARTICIPATE IN THE STUDY?
You may be eligible to participate if you meet the following criteria*:
- 30-75 years old
- Diagnosed with FTD-GRN (confirmed with a genetic test)
- Have a caregiver who is able to support you (including attending study visits) for the duration of the study (5 years and 3 months)
*Other criteria will also apply.
WHAT IS THE STUDY TREATMENT?
The study treatment in this clinical research study is AVB-101, an investigational one-time therapy designed to deliver a functional copy of the progranulin (GRN) gene directly to the brain, thereby potentially restoring progranulin levels and stopping disease progression in patients with FTD-GRN. AVB-101 is administered using a neurosurgical procedure directly to the thalamus at expert neurosurgical centers in Europe and the United States.
WHAT DOES STUDY PARTICIPATION INVOLVE?
Study participation involves visiting a clinical trial site at different points during the study for screening and follow up, and visiting an expert neurosurgical site for one-time dosing of AVB-101. Travel reimbursement for study visits will be provided.
INFORMATION ON CLINICAL SITES AND CONTACT INFORMATION
Study site locations and further study details are available at: https://clinicaltrials.gov/study/NCT06064890
You can also contact AviadoBio by email at clinicaltrials@aviadobio.com
VES001 is an experimental treatment that was designed to restore normal levels of the progranulin protein (PGRN) in the brain. The study will collect data to evaluate how safe VES001 is, as well as how well the body handles it (pharmacokinetics), what it does in the body (pharmacodynamics), and whether people can handle taking it without too many side effects (tolerability). In this phase 1 study, all participants will receive the experimental treatment.
Who is eligible?
- Men and women aged 18 to 75 years who are asymptomatic carriers of a GRN variant
- Body mass index between 18 and 32 (kg/m2), with minimum weight of 50 kg
- Using birth control during the study and for at least 90 days after the last dose
- Does not smoke more than 10 cigarettes per day and agrees not to smoke during the study period
- Does not have any known neurological disease, head trauma or loss of consciousness
- Does not have a history of cancer within the last 5 years, abnormal lab tests for infection diseases (Hepatitis B, Hepatitis C, and/or HIV), or any medical condition that could interfere with the study
- Does not have an abnormal ECG, abnormal heart rate or heart rhythm (e.g. atrial fibrillation), personal or family history of long QT syndrome or sudden death, untreated high or low blood pressure
- Is not currently pregnant, breastfeeding or planning to become pregnant
- Does not have severe allergic reactions to medication
- Has not donated blood or had significant blood loss recently
- Does not consume excessive caffeine (more than 8 cups per day)
- Has not participated in other research studies involving study treatment or devices
Interested individuals meeting the above requirements should contact the study coordinator for additional requirements and exclusions if interested in participating.
What will happen during the study?
Healthy participants who carry a GRN variant will receive 2 doses of the experimental treatment over the 16 week participation period. During the study period, there will be 8 visits. Blood, urine will be collected throughout the study period. In addition, spinal fluid will be collected through a spinal tap at three visits. Additional evaluations including ECG and neurological exams will be conducted as well.
What are they measuring to evaluate the experimental treatment?
- Side effects and reactions to the experimental treatment
- Changes in vital signs (pulse, blood pressure)
- Changes in electrocardiogram (ECG)
- Changes in physical and neurological examination findings
- Changes in Suicide Severity Rating Scale
- Changes in the concentration of the experimental treatment and other biomarkers in blood samples and spinal fluid
How long will participants be involved in the study?
Study participants will be monitored for 16 weeks.
Researchers at several sites across Canada, led by Dr. Simon Ducharme, are conducting a study to see if a medication named nabilone, taken by mouth, can help reduce restlessness and agitation in people with Frontotemporal Dementia (FTD), including bvFTD and PPA. They are inviting people who are thought to have FTD and are experiencing these challenging behaviors to participate. The aim is to see if this medication can provide some calm and comfort compared to a dummy pill (placebo) that doesn’t contain any medicine.
You, or someone you care for, may be able to take part if you/they:
- Are over 18 years of age
- Have symptoms of FTD
- Experience agitation, irritability, or aggression
- Have an available study partner
- Are living in Canada
For more information about the study and research sites participating in the study, visit https://clinicaltrials.gov/study/NCT05742698?term=NCT05742698&rank=1
Nabilone has not been approved for FTD by the FDA or any other health authority.
DNL593 is an investigational treatment currently being studied as a progranulin (PGRN) replacement therapy for FTD-GRN.
The Phase 1/2 study is now actively enrolling eligible participants in the US (Hospital of the University of Pennsylvania) and across 29 sites globally. More information on the study site locations and criteria can be found on ClinicalTrials.gov. Individuals with questions regarding clinical studies should speak with their healthcare provider.
DNL593 is an investigational medicine and is not approved by any Health Authority. No conclusions regarding safety or efficacy can be made.
CervoMed is seeking men and women aged 40-85 with a diagnosis of nonfluent variant primary progressive aphasia (nfvPPA) for an exploratory study to evaluate the effect of a medication called neflamapimod.
What will I be asked to do if I participate?
- Attend an initial screening visit at a study site to learn more about the study and determine if you qualify.
- Visit the study site 6 times over the first 24 weeks for study activities, including memory and language testing, speech assessments, and blood draws.
- Take the study medication by mouth three times a day for 24 weeks.
- After the first 24 weeks, be randomly assigned to continue the study medication or receive placebo (no active medication) for the next 12 weeks.
Am I Eligible? – the below listed criteria will be evaluated by the study doctor
- Must have a clinical diagnosis of nfvPPA with at least one of the core features:
- Difficulty forming grammatically correct sentences (agrammatism)
- Effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech)
- At least 2 of the following 3 features must also be present:
- Trouble understanding long or complex sentences
- Ability to understand single words is mostly intact
- Knowledge of objects and their use is mostly intact
- Scores indicating mild cognitive or language changes on standard assessments (Global CDR® plus NACC FTLD score of 0.5 or 1; language domain score of 0.5, 1, or 2)
- Normal or corrected vision and hearing so participants can complete study tasks
- Fluent in English
- Must have a reliable study partner who can attend all visits and read, write, and understand English
To learn more, please contact the study location nearest to you:
Mayo Clinic (Rochester, Minnesota)
Study Coordinator (Makayla Kelleher)
Phone: 507-293-0903
Email: kelleher.makayla@mayo.edu
Ohio State University (Columbus, Ohio)
Study Coordinator (Emily Poland)
Phone: 614-293-5183
Email: emily.poland@osumc.edu
University of Pennsylvania (Philadelphia, Pennsylvania)
Study Coordinator (Alexandra Tavani)
Phone: 215-662-2060
Email: alexandra.tavani@pennmedicine.upenn.edu
Full study details can be viewed on ClinicalTrials.Gov.
Baycrest is looking to recruit older adults diagnosed with Primary Progressive Aphasia (PPA) to participate in a clinical trial sponsored by the National Institute of Health.
Cognitive neurologists, Dr. Howard Chertkow and Dr. Carlos Roncero, are exploring the use of a brain stimulation technique called transcranial direct current stimulation (tDCS) to decrease symptoms of aphasia when combined with speech therapy. Previous studies have shown that this type of brain stimulation can stimulate the growth of nerve cells in the brain and improve communication abilities between neurons.
What is tDCS?
Transcranial direct current stimulation (tDCS) is a non-invasive process performed using a portable device during which light electrical currents, generated by electrodes placed on the skull, stimulate a specific region of the brain. This is NOT electroshock. In previous studies, tDCS has been shown to improve symptoms of neurological disorders, such as depression, stroke, Parkinson's disease, chronic pain, insomnia, schizophrenia and aphasia.
How do you benefit from participating?
- Receive an experimental treatment that may reduce your current aphasia symptoms, especially for naming and spelling
- You will be involved in a clinical trial whose long-term goal is determining if this form of stimulation can become standard
- You will contribute to scientific research that may help future generations by increasing knowledge about aphasia
Am I Eligible to Participate?
- Have a diagnosis of Primary Progressive Aphasia (NOT Semantic Variant PPA)
- Between the ages of 50 and 80
- Able to communicate in English
- Have no history of neurological or psychiatric disorders, except depression or anxiety
- Be able to learn and follow directions.
- During your first visit, you will complete a cognitive assessment to determine your eligibility for the study.
What Will This Research Involve?
It will take approximately five months to complete this study, however, therapy sessions take place only in the first and final month, with a 3-month break between these months.
During the active months, there will be 15 speech therapy sessions and 5 evaluations sessions to determine your performance and related improvement. You will also undergo 3 MRI scans.
One of these therapy months will also have tDCS administered while receiving speech therapy, while the other month will administer SHAM stimulation, which is a placebo condition. Results from each round will be compared to determine if the stimulation lead to more improvement than the placebo condition.
Compensation
There are no costs to you for participating in the study other than your time. Participants will also be paid for travel costs as TTC reimbursements or parking passes.
Interested in Participating?
For more information and details on how to participate, please contact:
Alice Zhang
T: 647-207-5039
E: tdcscoordinator@gmail.com
Baycrest Academy for Research and Education is seeking participants for a new study to explore the potential of transcranial direct current stimulation (tDCS) for improving walking ability in people living with PSP or CBD.
Participants will be primarily asked to walk while receiving transcranial direct current stimulation (tDCS) with evaluations before and at the end of stimulation sessions, as well as two weeks later, to assess if these stimulation sessions lead to sustained improvement in walking ability. Other tasks will also be done during stimulation, and during evaluations, to examine if these sessions can also produce improvements in speech and general cognitive function.
Previous research has shown that tDCS, when paired with targeted therapy, can produce benefits across multiple domains—such as memory, language, general cognition—and can also enhance motor abilities, including balance.
What to Expect
Participants will receive two rounds of stimulation, 2 months apart.
Each round consists of 12 stimulation sessions for three weeks (Monday - Thursday), with a follow-up evaluation two weeks later.
Parking will be compensated.
For More Information
Please contact tdcscoordinator@gmail.com.
Recruiting March 2024 - March 2028
Who?
Individuals with a diagnosis of Primary Progressive Aphasia and their Communication Partners.
Why?
Researchers at the University of Chicago - Healthy Aging & Alzheimer's Research Care Center want to develop evidence-based strategies to maximize communication participation and quality of life and minimize burden of persons with PPA and their communication partners.
Where?
All components of the study take place remotely via telehealth.
How Long?
Over the course of 18 months, participants in the study will be involved in:
- Up to 10 evaluations with a licensed speech language therapist
- Up to 25 therapy sessions with a speech language therapist
- Exercises through a web-application
There are no costs to participate in this study. Compensation will be provided.
If interested, contact the study team for more information:
- Phone: (773) 795-1111
- Email: cbtrial@uchicago.edu
- Website: haarc.center.uchicago.edu
The Gamma-Induction in FrontoTemporal Dementia (GIFTeD) Study is a randomized, double-blind, placebo-controlled clinical trial to assess the safety, tolerability, and efficacy of using transcranial alternating current stimulation (tACS) in FTD. Principal Investigator is Dr. Emiliano Santarnecchi at Massachusetts General Hospital and Harvard Medical School in Boston.
tACS is a safe, noninvasive way to stimulate the brain using weak electric currents. Electrodes are placed into a cap that is worn on the head. By synchronizing brain activity, tACS is aimed at increasing brain metabolism and boost cognitive performance, with the effects outlasting the stimulation period. The intervention is done 1 hour a day on weekdays for 6 weeks, for a total of 30 treatments.
There also is an in-person checkup at 3 months and a phone follow-up at 6 months. Additional tests include neuropsychological assessments, FDG-PET scans, MRI scans, EEGs, and blood draws before and after treatment.
The study seeks to enroll 50 people who have been diagnosed with behavioral FTD (bvFTD).
To be eligible, participants must be between 40 and 80 years old, stable on their medications for 30 days, and able to comply with study procedures.
There are 2 sites: Massachusetts General Hospital in Boston and the Santa Lucia Foundation in Rome, Italy.
For more information, contact Julianne Reilly at 617-667-9088 or jrreilly@mgh.harvard.edu or visit the study listing on ClinicalTrials.gov.
Researchers at Queens College, CUNY and Hunter College, CUNY are seeking participants with semantic or logopenic variants of Primary Progressive Aphasia (PPA) for a paid research study. We are exploring whether combining a personalized intensive language treatment with a computer-based memory training program implemented at home will result in lasting improvements in word finding and language skills.
Participation options:
In-person: Sessions take place at Queens College (65-30 Kissena Blvd, Queens, NY) or Hunter College (425 East 25th Street, Manhattan, NY).
Remote: Sessions can also be completed virtually through Zoom/telehealth from your home.
Study details:
26 sessions over 3 months
Each session lasts about 1 hour
Participants will receive $17 per session ($442 total)
Additional information:
Participants will be asked to sign a consent form explaining the study and their rights in more detail.
Individual progress feedback will be provided, and participants will receive a plain-language summary of study findings once results are analyzed.
If you are interested in participating, or know someone who may be interested, please email: avoge@hunter.cuny.edu
Hispanic/Latino People Living with Memory Challenges and Study Partners Needed
Researchers from Indiana University recently received funding from the American Federation for Aging Research to test whether a Green Activity Program can be done and if Hispanic/Latino people living with memory challenges enjoy it. The program was designed with people living with memory challenges who identified as Hispanic/Latino and care partners, local outdoor activity organizers, and healthcare providers. The goal of the program is to help people stay active and improve their health and well-being by participating in nature activities they enjoy.
The program is led by occupational therapist who help people do the activities they want and need to do to participate in daily life. In this study, they will work with you and a person you identify to support you in the study, called a study partner, to teach you strategies to help you safely participate in nature activities you enjoy. They will also work with you to add the activities to your daily routine.
They are currently looking for Hispanic/Latino people living with memory challenges and their study partners, to participate in this research study.
Participants living with memory challenges will complete surveys and a brief phone interview, wear an activity tracker on their thigh for two weeks, participate in an occupational therapy evaluation and a personalized green activity plan. They will also participate in 4-8 in-person program sessions in a location of their choosing, and two phone or Zoom check-ins. Total time participating is 33 hours over 17 weeks. Study partners will participate in the occupational therapy evaluation and at least one session for a minimum participation of 5.5 hours over 9 months. If you do not have a study partner, you can still participate. If you are eligible and agree to participate, you will be compensated $25 for pre and post testing for a total of $50. Learn more about eligibility and how to sign up by clicking the link below.
To be eligible, participants living with memory challenges must:
People living with memory challenges:
- 60 years or older
- Identify as Hispanic/Latino
- Have memory challenges or difficulties thinking
- Speaks Spanish or English
- T-MoCA scores 13-19
- Capacity to provide informed consent
- Have access and ability to respond to the telephone (mobile or landline)
Study partner
- 18 years or older
- Identify as Hispanic/Latino
- Speaks Spanish or English
- Identified by a person living with memory challenges as a person they feel comfortable with who they would like to join them in the study. The study partner’s participation is optional.
If you are interested in participating or to learn more, please contact Dr. Lassell at (812) 855-2395 or email at gapbronx@iu.edu. Your call will not obligate you to take part in the research study. Your participation is completely voluntary, and you can change your mind at any time.
Duke University is recruiting patients with Alzheimer’s, frontotemporal dementia, mild cognitive impairment, Parkinson’s, Down syndrome, multiple sclerosis, PTSD, concussion, traumatic brain injury, dementia, or other neurodegenerations fora study to take noninvasive pictures of the retina in your eyes.
No x-rays, no eye drops and no eye contact. Compensation for time/travel.
You may be eligible for this research study if you:
- Do not have a significant head tremor
- Are not in a wheelchair
- Have not had prior retina surgery
- Are willing to have some undilated pictures of your retina
Email iMIND@duke.edu for further details! Please feel free to forward this message to friends or family members who may be interested in participating in this study. The study visit takes 30-45 minutes. No charge to you or your insurance. The study is being conducted at the Duke Neurological Disorders Clinic, 932 Morreene Rd, Durham, NC 27705.
Researchers at the University of Pennsylvania are conducting a study to determine whether a form of non-invasive brain stimulation called transcranial direct current stimulation (tDCS) in combination with a naming and spelling intervention (NASP) can improve the language abilities of people with primary progressive aphasia (PPA).
tDCS uses a mild electrical current, about the same strength as a 9-volt battery, to stimulate regions of the brain from outside the head. This is performed using two small electrodes placed inside saline-soaked sponges held on the scalp using an elastic band. NASP is a speech intervention that focuses on improving oral naming and writing.
You may be eligible to participate if you are:
- 50 – 90 years old
- Right-handed
- Proficient in English
- Have aphasia due to logopenic or nonfluent Primary Progressive Aphasia
Participants will be compensated for their time and travel.
For more information, please contact the Laboratory for Cognition and Neural Stimulation at 215-964-2502 or braintms@pennmedicine.upenn.edu.
Sleep difficulties are common in persons living with memory loss, cognitive changes, or dementia, and their care partners and can negatively impact both mental and cognitive health. We invite you to participate in the SLEEPMATE Study—a virtual research study focused on improving both their sleep. This study aims to test whether a 6-week tailored Cognitive Behavioral Therapy for Insomnia program, delivered via videoconferencing, is feasible, acceptable, and effective in improving sleep.
To help determine if you qualify, please take a moment to answer these brief questions:
- Are you a care partner (e.g., spouse, sibling, adult child) to someone with memory loss or cognitive changes? Or are you someone with memory loss/cognitive changes who has a care partner?
- Do you both live in the same household?
- Do you both experience sleep difficulties?
- Are you willing to wear an activity watch (similar to a Fitbit) during the study?
If you answered “yes” to these questions, you both may be eligible to participate.
If eligible, both members of the care pair will receive compensation for their time and participation.
Email: drbrewsterlab@emory.edu
Phone: 404-712-9164
Website: http://www.mindatrest.org
Baycrest is looking to recruit older adults diagnosed with Primary Progressive Aphasia (PPA) to participate in a clinical trial sponsored by the National Institute of Health.
Cognitive neurologists, Dr. Howard Chertkow and Dr. Carlos Roncero, are exploring the use of a brain stimulation technique called transcranial direct current stimulation (tDCS) to decrease symptoms of aphasia when combined with speech therapy. Previous studies have shown that this type of brain stimulation can stimulate the growth of nerve cells in the brain and improve communication abilities between neurons.
What is tDCS?
Transcranial direct current stimulation (tDCS) is a non-invasive process performed using a portable device during which light electrical currents, generated by electrodes placed on the skull, stimulate a specific region of the brain. This is NOT electroshock. In previous studies, tDCS has been shown to improve symptoms of neurological disorders, such as depression, stroke, Parkinson's disease, chronic pain, insomnia, schizophrenia and aphasia.
How do you benefit from participating?
Receive an experimental treatment that may reduce your current aphasia symptoms, especially for naming and spelling
You will be involved in a clinical trial whose long-term goal is determining if this form of stimulation can become standard
You will contribute to scientific research that may help future generations by increasing knowledge about aphasia
Am I Eligible to Participate?
- Have a diagnosis of Primary Progressive Aphasia (NOT Semantic Variant PPA)
- Between the ages of 50 and 80
- Able to communicate in English
- Have no history of neurological or psychiatric disorders, except depression or anxiety
- Be able to learn and follow directions.
- During your first visit, you will complete a cognitive assessment to determine your eligibility for the study.
What Will This Research Involve?
It will take approximately five months to complete this study, however, therapy sessions take place only in the first and final month, with a 3-month break between these months.
During the active months, there will be 15 speech therapy sessions and 5 evaluations sessions to determine your performance and related improvement. You will also undergo 3 MRI scans.
One of these therapy months will also have tDCS administered while receiving speech therapy, while the other month will administer SHAM stimulation, which is a placebo condition. Results from each round will be compared to determine if the stimulation lead to more improvement than the placebo condition.
Compensation
There are no costs to you for participating in the study other than your time. Participants will also be paid for travel costs as TTC reimbursements or parking passes.
Interested in Participating?
For more information and details on how to participate, please contact:
Alice Zhang
T: 647-207-5039
E: tdcscoordinator@gmail.com
The upliFT-D Study, sponsored by Passage Bio Inc., is an interventional study investigating the safety and tolerability of a gene therapy (PBFT02) for persons with frontotemporal dementia (FTD) who have a mutation in the progranulin gene (GRN). Gene therapy aims to replace the gene that is not working properly with a version of the gene that works normally.
Participants will receive 1 dose of PBFT02. Additional assessments include blood tests, medical exams, questionnaires, brain imaging, and lumbar punctures.
There is a 2-year main commitment with a 3-year safety extension for a total of 16 visits over 5 years.
Eligible participants are
- diagnosed with FTD-GRN,
- between 35 and 75 years old,
- experience FTD symptoms,
- have a reliable study partner, and
- live in the community.
Travel reimbursement is available.
For more information, visit the study listing on ClinicalTrials.gov.
Veri-T is a phase 1 randomized, double-blind, placebo-controlled study of the safety and efficacy of the drug verdiperstat in persons with semantic variant primary progressive aphasia (svPPA), also known as semantic dementia.
The study will test the effects of verdiperstat on cerebrospinal fluid (CSF) proteins, brain magnetic resonance imaging (MRI), and cognitive tests. The drug or placebo is taken orally twice a day for 24 weeks.
Monthly visits to a study site are required for 6 months. Tests are done before and after.
To be eligible you must:
- be between 18-85 years of age;
- have a diagnosis of svPPA;
- be willing to undergo two lumbar punctures;
- be able to swallow pills; and
- have a study partner who spends at least 5 hours a week with you and can accompany you to study visits.
Target enrollment is 64 people. There are 5 study sites across the United States recruiting: University of California San Francisco; University of Pennsylvania; Northwestern University; Houston Methodist Hospital; and Mayo Clinic Rochester.
If you are interested in learning more about this study, please email ucsfteamverit@ucsf.edu or visit ClinicalTrials.gov.
Caregiver Studies
CAREVIRTUE PLANNER STUDY
Researchers from Indiana University in collaboration with a small business, CareVirtue, recently received funding from the National Institutes of Health (NIH) to study whether a personalized legal and financial planning platform for family caregivers of people living with Alzheimer’s disease and related dementias is useful. They are currently looking for family caregivers to participate in this research study.
The study asks you to use CareVirtue Planner, a financial and legal planning platform that provides financial and legal planning education and support to family caregivers of people living with dementia on a laptop or desktop computer for 3 months, and to answer questions about your experience using CareVirtue Planner during and after your time using the platform. If you agree to participate, you will be compensated up to $150 and will be able to use CareVirtue Planner without cost for one year. At the end of one year, you will be able to opt into the paid version of CareVirtue.
To be eligible, participants must:
- Be a self-identified primary caregiver status for someone with Alzheimer’s disease or related dementias
- Have access to the internet and access to a laptop or desktop computer
- Be over the age of 18
If you are interested in participating or to learn more, please contact our study team led by Dr. Werner at werneriu@indiana.edu. Your email will not obligate you to take part in the research study. Your participation is completely voluntary, and you can change your mind at any time.
CAPELLA UNIVERSITY STUDY
|
Priyanka Dinodiya is conducting a doctoral research study at Capella University to explore the challenges caregivers face and how to improve resources and support systems for those caring for individuals with frontotemporal degeneration (FTD) and apathy. Who Can Participate?
Purpose of the Research: The purpose of this research is to understand the challenges and coping mechanisms of caregivers who provide care to individuals with FTD and apathy. Your insights will help improve support systems for caregivers and individuals affected by this condition. Study Procedures: As a participant in this study, you will be asked to take part in a semi-structured interview, where we will discuss your caregiving experiences, challenges, and the impact of caring for someone with FTD and apathy. Interviews will take place via Zoom and are expected to last 60-90 minutes. Your responses will remain confidential. Time Commitment: The interview will take 45- 60 minutes. There are no other time commitments beyond the interview. For more information or to check eligibility, please email Priyanka Dinodiya at pdinodiya@capellauniversity.edu. |
CARE-MOVE STUDY
Drexel University is offering a unique opportunity to support your well-being through a creative and mindful approach with an online mindful movement study for dementia care partners.
Who can participate?
- Age 18 or older
- Primary caregiver for an individual with dementia
- Access to internet connectivity
Why participate?
- Enhance Self-Care and Stress Management
- Improve Mood and Well-Being
- Strengthen caregiving skills
- Connect with other care partners in a supportive environment
- Contribute to research
Program Details:
- Weekly 60-minute online sessions for 10 weeks
- Live, interactive sessions via video conferencing
- Complete surveys
- Receive $120 upon completion of the study
For more information or to join the study, please call (267) 359-5929 or email mbmresearch@drexel.edu.
Caregiver Bootcamp is a new program from the Nell Hodgson Woodruff School of Nursing at Emory University for caregivers of people recently diagnosed with Alzheimer’s Disease, Frontotemporal Degeneration (FTD), or another type of dementia.
What is Involved?
5 Online Sessions on Zoom
3-4 Short Interviews
Note: you will be compensated with a gift card for each interview.
Why Join?
Participants will gain an understanding of their new caregiver role, learn practical strategies, get connected to resources, and build confidence for the journey ahead.
Interested?
Visit this link to sign up.
For any questions, call or email the study team at 404-544-9916 or boot.camp@emory.edu.
The University of Colorado Multidisciplinary Center on Aging are seeking participants for a research study about caregiver perspectives.
Who is eligible to participate?
- Aged 18 or older
- Providing care to a person living with dementia or FTD
Details of the Study:
- Eligible participants who enroll in the study will be asked to answer survey questions at up to 2 time points about 1 month apart.
- Surveys take 30-45 minutes.
- Participants are eligible to receive a $25 gift card.
The survey asks about your demographics, perspective on dementia or FTD disorder care quality, and experiences as a support person for someone with dementia or an FTD disorder.
For more information, please visit www.bit.ly/CaregiverVOICE or contact: dementia.care@cuanschutz.edu or 303-724-8466.
Researchers at the University of Nebraska at Omaha are looking for individuals who are 50 years and older that are currently serving as an unpaid, family caregiver to an individual with mild cognitive impairment, Alzheimer’s disease, Frontotemporal dementia, Lewy body dementia, or vascular dementia. The study will involve one virtual visit for 90 minutes and will be conducted over the internet. Compensation for study participation is available. The experiment involves completing online questionnaires/interview and computer tasks. To be eligible for the study, you must be 50 years of age or older and currently serving as an unpaid family caregiver to an individual who is 40 years of age or older with mild cognitive impairment, Alzheimer’s disease, Frontotemporal dementia, Lewy body dementia, or vascular dementia, for 5 hours a week or more, for at least 6 months. In addition, to be eligible you should have comprehension of written and spoken English, and have completed a minimum of two years of high school or higher. You are not eligible for the study if you have a diagnosis of a neurological or psychiatric disease (e.g., stroke), history of drug abuse, vision, hearing, cognitive, or motor difficulties, or if you are currently pregnant.
For more information about the study, please contact: Naomi Adjei at the Aging Brain and Emotion Lab (402-554-5961) in the Department of Gerontology at the University of Nebraska at Omaha or by email at (beadlelab@unomaha.edu)
COMPASSION COMPASS STUDY
FAMILY CAREGIVERS ONLINE SURVEY
Researchers at the University of Oregon are seeking volunteers for an anonymous online survey exploring the needs of individuals living with and caring for a family member with dementia. Participants may not benefit directly from taking the survey, but they would be contributing to research aimed at reducing difficulties associated with caregiving.
You may be eligible for this study if you:
- Are an unpaid caregiver for a family (or chosen family) member with dementia
- Have been caregiving for at least 2 months
- Live at home with the care recipient
- Are 18 years or older
Participants will:
- Complete a 30-minute survey
- Receive a $40 gift card
To learn more, please contact us at eatinglab@uoregon.edu or 541-346-7494.
Emory Caregiver Study
Dr. Emily Mroz and her team at the Frost Lab at Emory School of Nursing are seeking caregivers for people with an FTD disorder, dementia, or other memory changes to participate in in a virtual study.
Who can participate
Individuals who are:
- Over the age of 40
- Taking care of someone living with an FTD disorder, Alzheimer’s disease, dementia, or memory changes
- Started providing care in the last 1-3 years
If selected, your study session will be virtual. You will complete survey questions and share stories from caregiving. Your data will be kept private and the team will share findings with you.
To learn more about participating, contact Dr. Emily Mroz at her team at Mroz.lab@emory.edu or call 404-544.9645.
LEADing End-of-Life Care Conversations
University of Utah College of Nursing is looking for pairs who would like to complete advance care planning documents and discuss their care preferences with each other.
The study lasts 20 weeks, but only takes six hours of your time.
You can participate in the comfort of your own home using your laptop, desktop computer, tablet, or Chromebook.
Participants will be paid for their time.
WHO CAN PARTICIPATE
This study is for pairs (2 people).
The first person has changes in their memory or is in the early stages of dementia.
If you are this person, you may be eligible to participate if:
- You are age 50+
- You are interested in having conversations about and documenting your wishes for your future care
- You have noticed changes in your memory or thinking skills OR
- You have been diagnosed with mild cognitive impairment, Alzheimer’s disease or another type of dementia
The second person is the care partner, usually a spouse/partner, family member, or close friend.
If you are the care partner, you may be eligible to participate if:
- Your are the spouse/partner, family member, or close friend
- You are age 18+
- This study is conducted by Dr. Kara Dessel, University of Utah, College of Nursing.
To participate, email lead@utah.edu or visit this website.
Mayo Clinic Personalized Tool for AD/ADRD Caregivers Addressing Financial Management and Legal Planning-Phase II
The Mayo Clinic is seeking caregivers of individuals diagnosed with Alzheimer’s Disease and Alzheimer’s Disease Related Dementias.
This study is fully remote. You will participate in two virtual focus groups and complete some surveys and questionnaires that are accessible via computer.
You will be asked to:
• Complete surveys/questionnaires (20-30 minutes, before, during and after focus groups)
• Participate in 2 focus groups (2 x 2 hours each, 4 hours total)
• View and engage with a prototype website – FinLe website
• Provide informal input/feedback if requested (2-4 times, 20-30 minutes each)
If you are interested in participating, please complete this form.
Millennial Caregiver Study
Are you providing care for an older adult family member or friend?
Does that person(s) have changes in their thinking and/or memory?
Were YOU born between 1946-1964 or 1981-1996?
The University of Virginia Department of Neurology is inviting caregivers to a research study to help us better understand Millennial caregivers and how they may be different from other generations of caregivers.
The research study involves:
- Answering online questionnaires for about 1 hour (get
- $85)
Optional 90-minute focus group for some Millenials (get an additional $95)
To participate in the study go to: https://bit.ly/3xH8030
For more information, call Chris Sheehan at 434-924-9386 or email caregiving@uvahealth.org.
PREVENT ALL ALS STUDY
The PREVENT ALL ALS Study is recruiting known carriers of genetic variants such as the C9orf72 expansion, individuals who are close family members (children, siblings, parents) of someone affected by a genetic form of ALS or FTD, or close family members of an known carrier of an ALS/FTD gene. People with a strong family history of ALS and FTD may also qualify even if no prior genetic testing has been performed in the family.
PREVENT ALL ALS is part of the ALL ALS Research Consortium, an National Institutes of Health funded program bringing together researchers, government agencies, companies, and non-profit organizations to enhance our understanding of ALS and develop better treatments. This network spans 35 research sites across the country all working together to build the largest ever repository of samples and clinical data from ALS patients, controls, and genetically-at-risk individuals with the goal of making these samples and data available to ALS and FTD researchers across the country
Participation in the PREVENT ALL ALS Study involves completing annual in-person study visits and additional remote visits every 4 months for up to 3 years. During these visits, participants will undergo clinical assessments, including:
- neurological exams
- cognitive and behavioral screenings
- speech assessments
- digital self-reported questionnaires
- biofluid sample collection including blood collection
- cerebrospinal fluid collection up to once per year is an optional assessment
Genetic counseling is offered to all participants in the study and participants have the option to pursue confidential genetic testing for ALS genes though the research study if they desire. Individuals may also participate in the PREVENT ALL ALS Study without pursuing genetic testing.
Participants in this study will receive a small stipend for completing study visits ($50 for each in person visit, $30 for each remote visit, $100 for each lumbar puncture). Reimbursement for travel is not provided through the study.
These research efforts are expected to result in knowledge that can lead to more informative, targeted, and personalized drug development, taking the field one step closer toward the goal of halting, repairing, and/or preventing ALS and FTD.
More information about ALL ALS can be found on the ALL ALS website or by emailing the Patient Navigation Team at info@all-als.org.
Spousal Caregiver Study
The University of North Carolina at Wilmington is looking for participants who are spousal caregivers of partners with dementia or frontotemporal degeneration.
Criteria:
- Age 60+
- Currently a Primary Caregiver
- Has Been a Caregiver for the Past Six Months
- Speaks Fluent English
Do you find joy in helping your loved one? Or does it bring you stress? Both of these reactions are valid! The goal of this research is to examine why some spousal caregivers experience compassion fatigue while some don't.
Please click here to complete the survey.
STELLA-FTD (Support via Technology: Living and Learning with Advancing FTD)
Do you care for a family member with frontotemporal degeneration (FTD)? You may be eligible to participate in a research study that offers support, education, and resources for caregivers.
STELLA-FTD (Support via Technology: Living and Learning with Advancing FTD) is a program for family Care Partners that addresses the challenges that come with frontotemporal degeneration (FTD). This study is for anyone who cares for a family member with an FTD disorder.
STELLA-FTD is a completely online study. Participation can take up to 36 weeks.
Participants will complete:
- 8 small-group videoconference visits to learn how to manage challenges that come with FTD caregiving (each visit lasts about 1 hour and will be led by professional Guides)
- a weekly review of study materials and resources; one online survey with demographic questions about the participant and their family member
- brief surveys sent by email every week
- six longer surveys during the study period about mood, coping, and family member behaviors; and (optional) a focus group.
Gift cards up to $200 may be provided for completing all study activities.
TACAD STUDY
The University of Texas at Austin School of Nursing is looking for research participants to help reduce the burden of caregiving on the health of Asian American midlife women family caregivers of persons living with Alzheimer’s disease or other dementia.
You May Be Eligible If You:
- Are between 40 and 65.
- Can read and write English, Mandarin Chinese, or Korean.
- Live in the United States.
- Have access to Internet.
- Are a community-dwelling family caregiver of a person living with Alzheimer’s Disease or other dementia (clinical dementia rating greater than or equal to 1).
- Are providing at least 4 hours per day (on average) assistance to the person living with dementia.
Who We Are
Dr. Eun-Ok Im and her team at The University of Texas, Austin want to learn effectiveness of the newly developed program in improving health outcomes of Asian American midlife women who are family caregivers of persons living with Alzheimer’s disease or other dementia.
About the Study
TACAD study is an online study and does not involve medication nor travel.
TACAD is Technology-based Information and Coaching/Support Program for Asian American midlife women who are family Caregivers of persons living with Alzheimer’s Disease.
Compensation
Up to $150 will be provided during this 3-month study duration.
How can I contact the research team for further information?
If you have any questions or need more information, please visit https://tacad.research.nursing.utexas.edu or contact us using email tacad.help@austin.utexas.edu or call at 512-232-2323.
PRIMARY CAREGIVERS OF A LOVED ONE WITH DEMENTIA STUDY
Tissue Donation
Understanding of FTD has increased dramatically thanks to families who have been willing to donate tissue to research through brain donation when a loved one passes away. This is a complex and difficult decision for any family to make. If you are interested in learning more about tissue donation, please visit our Brain Donation page.
More places to Find Studies
- The Association for Frontotemporal Degeneration posts a table with studies currently recruiting volunteers for the FTD disorders, AFTD-clinical trials listing.
- CurePSP posts listings of studies for families affected by PSP and CBS/CBD.
- Michael J. Fox Foundation for Parkinson’s Research lists studies and can help match patients with CBD and PSP to trials using the FoxTrialFinder.
Together we can find a cure for ftd
The FTD Disorders Registry is a powerful tool in the movement to create therapies and find a cure. Together we can help change the course of the disease and put an end to FTD.
Your privacy is important! We promise to protect it. We will not share your contact information.
RESOURCES
Learn more about FTD disorders, caregiver support, and organizations that can help you and your family here.
FEATURED STUDY
upliFT-D Study
The upliFT-D Study, sponsored by Passage Bio Inc., is an interventional study investigating the safety and tolerability of a gene therapy (PBFT02) for persons with frontotemporal dementia (FTD) who have a mutation in the progranulin gene (GRN). Gene therapy aims to replace the gene that is not working properly with a version of the gene that works normally.
For more information, visit the study listing on ClinicalTrials.gov.
Other Ways to Help
Spread the Word
Ask your family and friends to join in fighting FTD/Spectrum
Learn about FTD research
Why learn about FTD